Iron Carbon diagrams over the Years

By:Cees van de Velde
First Edition: May, 2001
cvdv@home.nl
Year of publication :1997
Author :T.Mizoguchi, J.H.Perepezko, C.R.Loper,Jr.
Source :AFS Transactions 1997,pp.89-93.
Notes:
Summary:
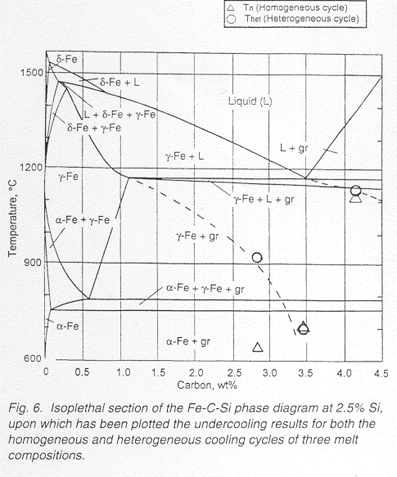
It has been demonstrated that a modification of the droplet technique can be effective in identifying the nucleation parameters in cast iron melts of both hypo- and hypereutectic composition. Primary austenite was observed to be a poor nucleant for both graphite and carbide,based on undercoolings of 220-430C below the eutectic. On the other hand, graphite has been demonstrated to be an effective nucleant for austenite at a modest undercooling of 20C. Effective nucleation requires consideration of both the requirements of lattice disregistry between the nucleant and the nucleating solid and the relative interfacial energy between the solids with respect to the melt. Interpretations cannot be based on lattice disregistry alone.
The droplet technique recorded a maximum undercooling of 620C in a 2.85% C. 1.91%Si alloy. This is the largest reported undercooling for Fe-C-Si alloys. At these large undercoolings, the microstructure formed as a carbon supersaturated metastable austenite. As much as 3.45% C was observed to be present in this supersaturated austenite, the greatest amount yet reported. Furthermore a new metastable carbide eutectic structure of about 9 wt%C formed with high undercoolings in the presence of austenite.
The droplet technique is a viable procedure to quantitatively characterize agents that are proposed as nucleants in commercial Fe-C-Si alloys. Their effectiveness in promoting the formation of austenite, graphite or carbide during solidification cannot only be determined, but can be determined quantitavely where the amount of undercooling obtained corresponds to the effectiveness of the nucleant. As a result, the cast iron industry now has a procedure whereby the true effectiveness of agents can be assessed.
Image
Back to image

